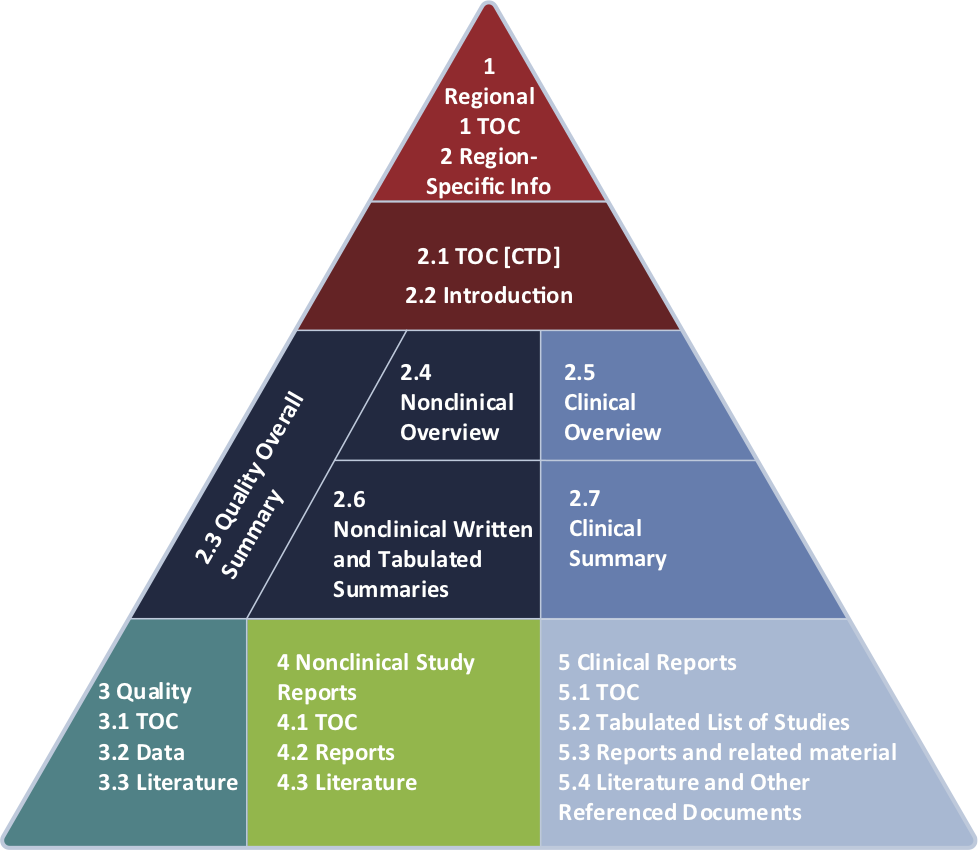
eCTD Compliance
WI is committed to providing excellence in both compilation and publishing services to our clients. Presenting health authorities with eCTD-compliant submissions is no longer a choice, it’s a requirement. We understand the importance of providing reviewers submissions they can easily navigate by applying all the technical specifications at both the document and submission level.
Services Offered
- Compilation: Compilation includes the following tasks: conversion to PDF, compiling all required components (eg, final draft of the MS Word document; TLFs; and appendices), inserting bookmarks, and inserting all internal hyperlinks. The final deliverable to the client is a functional, fully linked PDF.
- Publishing: Publishing includes the insertion of a PDF-rendered document into an XML backbone. This document is submission-ready to transmit through a health authority electronic submission gateway.
- Compilation/Publishing QC Review: QC review consists of review of each fully compiled document to ensure that all bookmarks and links are functioning correctly (compilation QC) and review of a fully published sequence to ensure all documents are placed appropriately in the XML backbone, external hyperlinks are functioning correctly, and required metadata are applied (publishing QC).
- Transmission: We have our own electronic gateway account or can use the account of our client to transmit documents to the applicable health authority.
- Storage: We have our own proprietary 21 CFR Part-11 compliant system, facilitating secure server-to-server transfer between WI publishers and our clients.
- eCTD Viewer: WI offers clients the option to include an eCTD viewer license for an easily accessible, transparent, and efficient regulatory review of electronic submissions.