We Understand Quality Regulations
Our CMC consultants have worked across the commercialization spectrum. They understand when you need to detail process development or formulation changes. They can review your Drug Master File and advise on your dossier strategy.
Regulatory & Technical Writing Areas of Specialization:
-
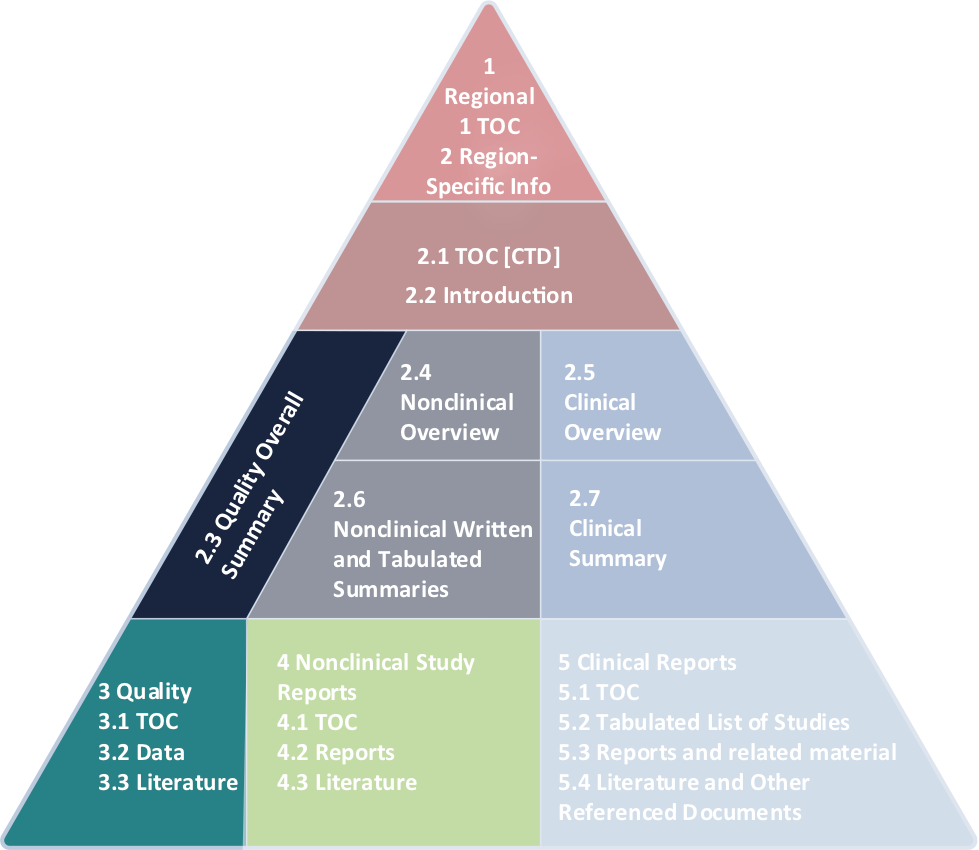
- Common Technical Document (CTD) and electronic Common Technical Document (eCTD) format and traditional (non-CTD) format
-
- Author and review in Module 2.3, Module 3, and appropriate Administrative Module 1 sections
- Experience with Octagon and Liquent eCTD template software
-
- Common Technical Document (CTD) and electronic Common Technical Document (eCTD) format and traditional (non-CTD) format
-
- Regulatory writing across regions for:
-
- Drug Master Files (DMF)
- Investigational New Drug Applications (IND)
- New Drug Applications (NDA)
- Abbreviated New Drug Applications (ANDA)
- Clinical Trial Applications (CTA), New Drug Submissions (NDS)
- Investigational Medicinal Product Dossiers (IMPD)
- Marketing Authorization Application (MAA)
-
- Regulatory writing across regions for:
-
- Regulatory writing across regions for Life Cycle and Maintenance of Business of approved products
-
- Supplements
- Annual Reports
- Variations
-
- Regulatory writing across regions for Life Cycle and Maintenance of Business of approved products
